On April 28, 2025, the eResearch Proposal Management system (eRPM) was updated to version 7.9 with several enhancements and bug fixes.
For Unit Research Administrators and Project Teams
PAF worksheet
(PAF Section 1. Project Information - Abstract)
Question 1.9* Project Abstract/Statement of Work is now a required field. This impacts any PAF that is in progress (editable). If you have a PAF and need to make changes, the Project Team will have to update the field. Question 1.10 Descriptive words is optional.
New ACR Type and Foreign Subrecipient Attestation Change
Attestations for NIH Foreign Subrecipients will no longer be collected via the Subcontract (SUBK) record's "Post a Comment to the Entire Project" activity within eRPM. Instead, the required attestation will be integrated into the “Approve ACR” workflow for new Award Change Request (ACR) types categorized as “Technical / Progress / Invention” Reports.
Within the ACR workflow, Principal Investigators (PIs) will be prompted to attest by selecting either “Yes” or “No” for each foreign subrecipient listed (see screenshot below). Responses will be automatically logged on the individual subaward records. If a PI selects “No,” the Office of Contract Administration (OCA) will be automatically notified. In such cases, OCA will initiate a formal termination notice to the SUBK organization for non-compliance, and will rescind the notice if the issue is rectified within 5 business days.
This process supports institutional accountability and aligns with NIH’s evolving expectations for oversight of foreign subrecipients under Research Performance Progress Reports (RPPRs).
Technical / Progress / Invention Report
Added a new Award Change Request Type, Technical / Progress / Invention Report, to request ORSP review, approval, and/or submission of a sponsor-required technical, progress, or invention report (e.g., NIH Annual or Final RPPR/Invention reports, Grants Online progress reports). A few notes about the new type:
- It cannot be selected in combination with another change type.
- It routes to ORSP after the PI approves it. (Unit approval is not required.)
- This type should not be used if ORSP does not need to sign or submit it.
Create ACR Form
New Technical / Progress / Invention Report Form
PI Approve ACR
Approve ACR activity
DURC-PEPP Policy Regulations
PAF worksheet - Section 5. Research Activity
Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential (DURC-PEPP) policy regulations go into effect on May 6, 2025 and will apply to all federally funded research.
PI/Project Teams must report, go through an assessment, and follow guidelines and reporting requirements (when DURC/PEPP applies).
A summary of steps include:
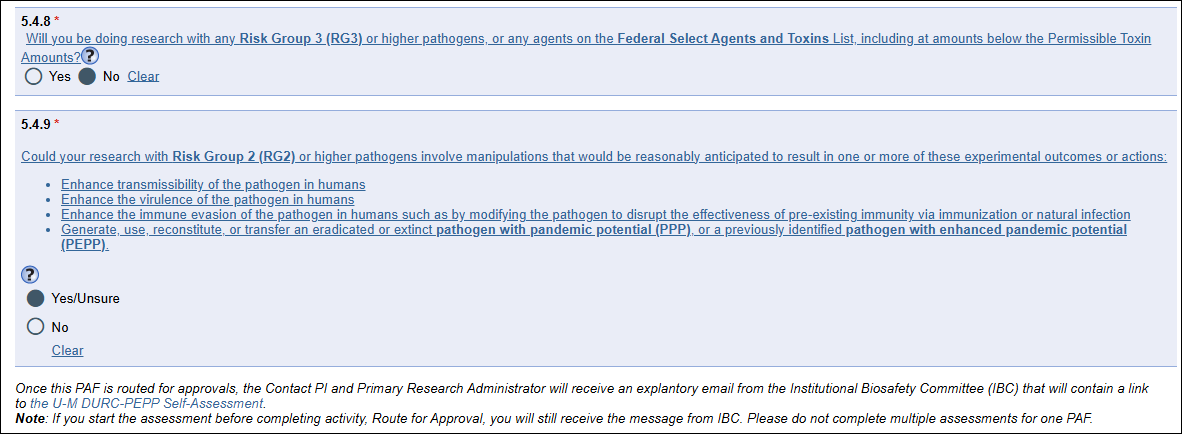
- Answer Institutional Biosafety Committee (IBC) questions on the PAF worksheet Section 5 Research Activity, or via Update Research Activity, Question 5.4 * Does this project involve research in a U-M laboratory with biological materials? [Yes/No]
- If “Yes”, then answer new required questions for 5.4.8 (Risk Group 3) and 5.4.9 (Risk Group 2). (see screenshot below).
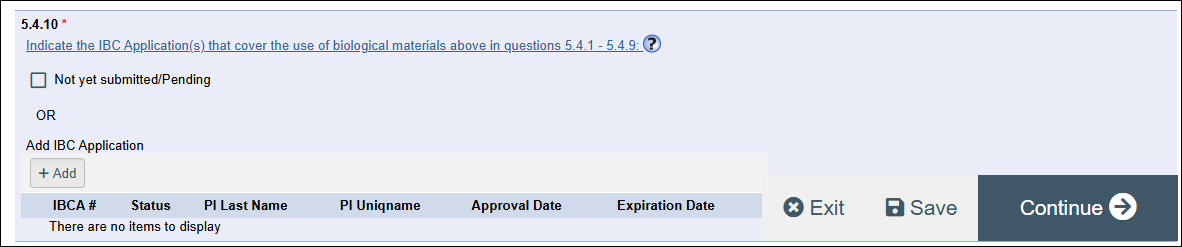
- If “Yes” to either, then answer question 5.4.10 IBC Applications.
- Once the PAF is routed for approval, the Contact PI and Primary Research Administrator will get an explanatory email from the IBC with a link to the U-M DURC-PEPP Self-Assessment for completion.
Refer to the DURC-PEPP Policy page on the Research Ethics & Compliance website for additional information.
PAF Research Activity, Questions 5.4.8, 5.4.9, and 5.4.10
Other Changes
PI & Project Team workspace
Enabled IT Managers to view TCPs, and ECAs and RISO categories of UFAs after they have approved them via a new "UFAs Assigned to You as IT Manager" list on the UFAs tab.
Award workspace SUBKs tab
Changed the "COI Review Required" column that displays on Awards > SUBKs tab to "Following U-M COI Policy".
For Central Offices
Export Controls Office
Removed access to “Manage Key Personnel” on Technology Control Plans (TCP).
Office of Research and Sponsored Projects (ORSP)
Enhancements in support of Realignment
ORSP-Coordinator Role
- Automated the Award Management Officer (AMO) assignment for Awards and Hardships
- Automated the Unfunded Contracts Officer (UCO) assignment for UFAs originating from campus.
ORSP-IT Role
- Provided the ability to add/update the designated PR (AMO, SCO, or UCO) for Sponsor Information in an Entity Profile to indicate the designated sponsor and flags the sponsor to indicate if the sponsor has "Firmly established terms and conditions".
- Provided the ability to add/update the PRs (AMO, SCO, CTO, UCO) for department IDs on the Department PR Assignment tab.
- Provided the ability to dynamically control the Pre-Award PAO Round Robin assignment criteria.
- Enhanced User Maintained Data to manage the PAOs and include or exclude from Round Robin assignments.
- Updated the assignment calculation to consider project complexity and review type.
- The definition of "Complexity" can be updated to choose predefined factors and thresholds to add or subtract from the calculation.
- For steps with screenshots, see the associated ORSP Complexity Job Aid.
Internal ORSP Requests
- Fixed a bug that accidentally cleared the PR when a document was added in the Internal ORSP Requests activity.
- Included the “Created date” of the Current Action Request on the associated PAF/AWD/MOD/ACR record.
- Improved load performance of “Internal Action Request” lists on the Unassigned Requests tab on the ORSP-PR workspace.
