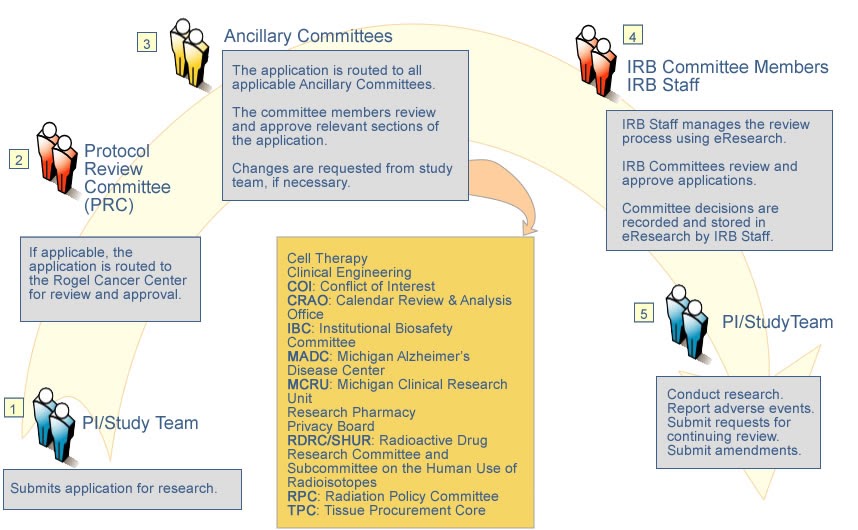
- The Principal Investigator (PI) submits a single application that is routed automatically to required committees for review. The IRB provides the final review.
- The study team (e.g., study coordinators, etc.) can assist the PIs in preparing applications.
- The application "smart form" guides you through the relevant information required for submission based on your responses to questions.
- Detailed questions and answers provide clarity/reduce ambiguity for reviewers.
- Data is validated (at time of entry or upon submission) to ensure quality and reduce the potential for changes required after submission.
- Reviews and approvals are conducted online.
- Email notifications include hyperlinks to specific submissions.
- Correspondence is maintained in the database for future reference.
- Changes are logged and time stamped for tracking purposes.
The PI on a submission is responsible for:
- Reviewing information entered by the study team (either online or via print version)
- Submitting the application electronically
- Re-submitting the application if changes are required by review committees
- Electronically submitting any adverse events, amendments, scheduled continuing reviews, and terminations.
You add Study Team Members in question 1.3 of the application. See Adding a Study Team Member for instructions.
From the Need to Accept Role section of My Inbox, click the name of the application or amendment. Then, click the Accept Role activity, and answer the questions. See Accepting Your Role on the Study Team for full instructions.
There are 45 sections in the application. Not all will be required for each study. The application smart form guides you through the relevant information required for submission based on your responses to questions. See the Spreadsheet of Application Questions for a list of each question and details about data entry and/or related system logic.
Once the study is approved, activities are available in the Study Workspace to submit:
- Adverse Events (AEs)
- Other Reportable Information and Occurrences (ORIOs)
- Scheduled Continuing Reviews
- Amendments
The Student column is displayed in the Study Team Members table and in question 1.3 to alert study team members that a member’s Student status makes appointment selection not required.
The IRB may ask a PI who shows up as a student but has a relevant appointment to select their appointment.
The customer must contact the Registrar’s Office to find out how to discontinue the active student program. (Note: MCommunity profiles indicate if one has a Student affiliation.) Once the academic program is no longer active, eResearch will automatically update the person’s Student column and Organization within 48 hours of the change being entered into M-Pathways Student Administration.
Make sure the correct appointment is selected on the study in question. If the appointment information available for selection is inaccurate, consult your department’s HR personnel. All information relating to a person’s affiliation with the university is pulled from M-Pathways Human Resource Management System (HRMS).